![]()
![]()
It is no coincidence that the isotope that has been studied the most in the Mossbauer field is Fe57. This iron isotope is of extreme biological importance. Approximately seventy-five percent of all experimental papers on Mossbauer Spectroscopy deal with Fe57. By pure luck, however, Fe57 happens to have almost ideal characteristics that make it the most easily observable Mossbauer nuclide. Our research revolves around Fe57 because even after all the papers and extensive study of this isotope, unknowns remain. Also, the applications of Mossbauer research of Fe57 are endless. The prime example is the better understanding of iron porphyrins.
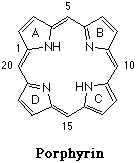
Porphyrins, are vital chemical compounds produced by all living organisms. Porphyrins are necessary for cell respiration. A porphyrin molecule is planar and looks like a circular ring made of four rings linked together, a fundamental skeleton is shown below. Atoms of various metals positioned in the center of the large ring distinguish different porphyrins. A much more familiar example is hemoglobin's prosthetic group, heme, which is an iron porphyrin. There is also Chlorophyll, the substance that gives green plants their color, which is a magnesium porphyrin. For in depth information on porphyrins see links on the Reference Page.
The Mossbauer experimental setup can be very simple and affordable, or very expensive and complex. The main three parameters that the Mossbauer spectroscopist can vary to study samples are: temperature, magnetic field, and the Doppler shift. Obviously, the most simple experimental setup consists of a zero-magnetic-field room-temperature spectrometer, and the most complex spectrometers are designed to run at cryogenic temperatures and with very high magnetic fields.
In Knox we currently work with two spectrometers with these specifications:
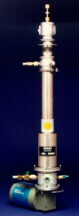
|
The CCC spectrometer: The Closed Cycle Cryostat Spectrometer |
The CCC is
shown in the picture above. Part of my previous research project
on Mossbauer spectroscopy during this past summer, was the construction
of the stand for the spectrometer apparatus. "The stand" had to meet
the most important Mossbauer requirement: "sufficient" vibration isolation.
Also, lead shielding around the source was needed. Here follows a
pictorial description of how "the stand" came to be:
 The
plan was to attach the CCC by its bottom part to a very heavy "cinder-block
and sand" base. This would damp the vibration from the motor into
the ground and also provide a very stiff support.
The
plan was to attach the CCC by its bottom part to a very heavy "cinder-block
and sand" base. This would damp the vibration from the motor into
the ground and also provide a very stiff support.


